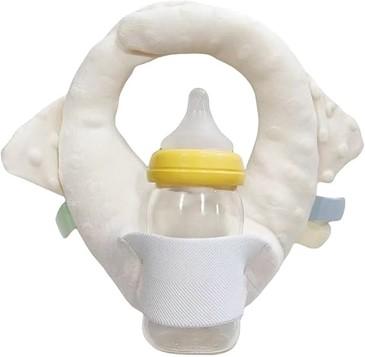
Health Canada warns that infant self-feeding devices sold on Amazon.ca pose choking and aspiration hazards
Summary
Immediately stop using the affected product and dispose of it.
Affected products
An infant self-feeding device is any device which allows caregivers to position a feeding bottle in a way that allows an infant to feed themselves unattended. Self-feeding devices can be in the form of:
- a device designed to support the bottle such that it can be stably placed either beside or on top of the infant, or
- a device that positions a bottle nipple in the infant’s mouth without requiring the infant to hold the supportive device while feeding.
This alert involves the following product:
- LQR Bottle Holder for Baby Self Feeding, Baby Bottle Holder Nursing Pillow, Twin Baby Essentials baby Bottle Holder Hands Free for Mam, Adjustable Baby Bottle Breastfeeding Must Haves (ASIN B0DHCZRG8P).
Issue
Infant self-feeding devices are banned in Canada.
These devices allow an infant to either hold the bottle and feed before developing the needed muscles to do this independently, or the bottles are situated in a way that makes it difficult for the infant to stop feeding. As a result, this product pose a choking or aspiration hazard to the infants using them, which can end in illness or death from aspirating the feeding liquid. When feeding, infants regurgitate small amounts of liquid food; therefore, they should be monitored at all times while feeding to ensure the caregiver can intervene if any concerning fluid intake or behaviours appear.
Feeding is traditionally interrupted periodically by the caregiver to burp the infant, which cannot be done if direct supervision is not provided. Unattended infant feeding practices are discouraged by Health Canada and Canadian professional medical associations as independent feeding should not be done until the child is ready.
What you should do
Consumers who have the affected product should immediately stop using it and dispose of the item.
Regularly check the Healthy Canadians Recalls and Safety Alerts database for dangerous products and take action to remove items of concern.
Health Canada would like to remind consumers to report any health or safety incidents related to the use of these products or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
Related links
Stay connected with Health Canada and receive the latest advisories and product recalls using social media tools.
Additional information
What is being done
Health Canada has banned infant self-feeding devices in Canada under the Canada Consumer Product Safety Act.
The product has been removed from sale online from Amazon.ca. Health Canada has contacted the foreign third-party sellers and is advising consumers to immediately stop using the infant self-feeding devices. Health Canada reminds consumers to learn about the risks of buying consumer products online (see Related Links above).
Amazon.ca reported that 105 units of the affected products were sold in Canada.
To date, no incidents or injuries related to this product has been reported to Health Canada.
Health Canada is committed to helping protect consumers from potentially dangerous consumer products. The Department regularly tests consumer products available for sale in stores or online and continues to monitor products to help keep consumers safe.
Details
Distributors
COLIN Store, Shenzhen, Guangdong, China
Taiyuanfengzhijiashangmaoyouxiangongsi (Taiyuan), Taiyuan City, Shanxi, China
瑞雪商贸-VV, Guangzhou, Guangdong, China
Place of Origin
China
Media and public enquiries
Media Enquiries
Health Canada
(613) 957-2983
Public Enquiries
(613) 957-2991
1-866 225-0709
Get notified
Receive notifications for new and updated recalls and alerts by category.