M-Eslon (morphine sulfate) ER capsules: One lot recalled as some bottles labelled as M-Eslon 30 mg may contain 60 mg capsules, which may pose an overdose risk
Summary
Check your M-Eslon (morphine sulfate) medication bottle or package. If your prescription is for 30 mg capsules and the bottle contains 60 mg capsules, or if you are unsure, do not take these capsules and return them to your pharmacy. If you or someone you are caring for is experiencing an overdose (symptoms are described below), call 911 immediately and seek immediate medical attention
Affected products
| Product | DIN | Lot number | Expiry date |
|---|---|---|---|
| M-Eslon (morphine sulfate) extended release capsules – bottles from the manufacturer are labelled as 30 mg | 02019949 | 43885 | 04/2026 |
Issue
Ethypharm Inc. is recalling one lot of M-Eslon (morphine sulfate) extended release (ER) capsules because some bottles labelled as M-Eslon 30 mg ER may contain 60 mg capsules. This issue could lead to patients receiving a 60 mg dose instead of their prescribed 30 mg dose, which could result in an overdose and pose serious health risks.
M-Eslon is a prescription opioid drug used by adults for long-term pain management when pain is severe enough to require daily, around-the-clock painkillers and other treatment options are not able to treat the pain.
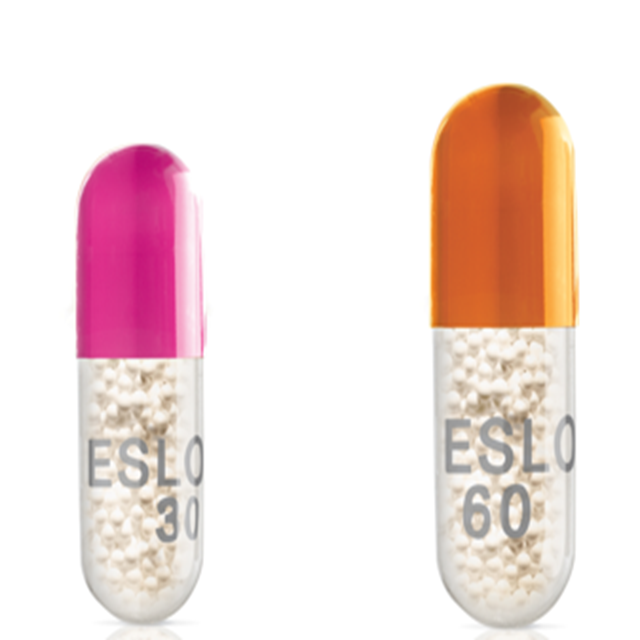
The 60 mg capsule has "60" and "M-ESLON" printed on it. The capsule has an opaque orange cap and a clear bottom.
The 30 mg capsule has "30" and "M-ESLON" printed on it. The capsule has an opaque pink cap and a clear bottom.
Taking too much morphine or suddenly increasing the dose could potentially lead to an overdose, which can be life-threatening. Symptoms of morphine overdose may include: confusion, dizziness, drowsiness, difficulties waking up, small (pinpoint) pupils, slow heartbeat, slow and difficult breathing, cold and clammy skin, fainting, seizure, coma and death.
Health Canada is monitoring the company's recall and investigation, including its implementation of corrective and preventive actions to stop this issue from reoccurring. The Department will inform the public if any new health risks are identified.
What you should do
- Check your M-Eslon (morphine sulfate) medication bottle or package. If your prescription is for 30 mg, the capsules should be pink and clear. If it contains 60 mg capsules (orange and clear), or if you are unsure, do not take these capsules and contact your pharmacy immediately. Your pharmacist will check the capsules and provide you with a replacement if needed. Return the affected product to your pharmacy for proper disposal.
- Call 911 immediately and seek immediate medical attention if you or someone you are caring for is experiencing an overdose (e.g., slow breathing, drowsiness, or difficulties waking up).
- If an overdose is suspected, administer naloxone nasal spray or injection without delay. Give a repeat dose after 2 to 3 minutes if the person has not woken up or their breathing has not improved.
- Contact your health care professional if you have taken the wrong dose of your medication and have any health concerns.
- If you have questions about this recall contact Ethypharm Inc. by calling 1-855-694-0151, or by emailing customerservice@valeopharma.com.
- Report any health product-related side effectsor complaints to Health Canada.
Additional information for health professionals:
- Health care professionals, such as pharmacists, should check bottles labelled as M-Eslon (morphine sulfate) 30 mg extended release (ER) capsules before dispensing to make sure they do not contain M-Eslon 60 mg ER capsules. Report any unusual bottles or other issues to the company and to Health Canada.
Additional information
Details
Media and public enquiries
Health Canada
613-957-2983
media@hc-sc.gc.ca
Public Enquiries:
613-957-2991
1-866-225-0709
info@hc-sc.gc.ca
Get notified
Receive emails about new and updated recall and safety alerts.