Three lots of JAMP Valproic Acid Oral Solution recalled
Summary
Do not stop taking your medication. Speak with your pharmacist as soon as possible to check if your product is affected by the recall and to obtain a replacement, if needed. If you cannot get to your pharmacy right away, check your product for crystals and ensure that no crystals are present in the dose before taking it or giving it to someone under your care.
Affected products
| Product | DIN | Lot | Expiry |
|---|---|---|---|
| JAMP Valproic Acid Oral Solution 250 mg/5 mL | 02532441 |
|
2025-10 |
Issue
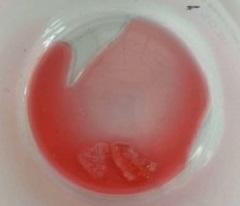
JAMP Pharma Corporation is recalling three lots of JAMP Valproic Acid Oral Solution after receiving complaints of large solid crystals that do not dissolve when the bottles are shaken, and that are difficult to break apart.
The company has indicated that the crystals are sucrose (a type of sugar). The crystals may pose a choking hazard, especially in young children or people with difficulty swallowing.
JAMP Valproic Acid Oral Solution is a prescription drug usually taken by adults and children two years of age and older to control epilepsy, a disorder of the brain that causes seizures.
Missing doses of valproic acid can lead to seizures, which is a very serious medical event. It is therefore essential that patients taking an affected product return it to the pharmacy as soon as possible for replacement and that they do not skip any doses. Patients should continue to take their medication until it can be replaced.
Health Canada is monitoring the company's recall and investigation, including its implementation of corrective and preventive actions to prevent this issue from reoccurring. The Department will inform the public if any new health risks are identified.
What you should do
- Do not stop taking this medication or delay taking a scheduled dose, as valproic acid is an important drug for preventing seizures.
- Speak with your pharmacist as soon as possible. Your pharmacist will check if the product is affected by the recall and provide a replacement if needed.
- Do not ingest the crystals. If you cannot get to your pharmacy right away, check your product for crystals and ensure that no crystals are present in a dose before taking it or giving it to someone under your care.
- Contact a health care professional immediately if you or someone you are caring for experiences serious side effects.
- Contact JAMP Pharma Corporation by calling toll-free at 1-866-399-9091, extension 501, or by email at custjamp@jamppharma.com, if you have questions about this recall.
- Report any health product-related side effects or complaints to Health Canada.
Additional information
Details
Media and public enquiries
- Media Enquiries:
- Health Canada
- (613) 957-2983
- media@hc-sc.gc.ca
- Public Enquiries:
- (613) 957-2991
- 1-866 225-0709
- info@hc-sc.gc.ca
Get notified
Receive notifications for new and updated recalls and alerts by category.