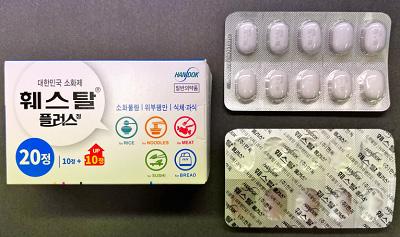
Unauthorized Korean-labelled Festal Plus tablets, seized from GD Health Town in Coquitlam, B.C., may pose serious health risks
Summary
Do not use this product. Consult a health care professional if you have used this product and have health concerns. Read product labels to verify that health products have been authorized for sale by Health Canada. Only buy prescription drugs from licensed pharmacies. Return products to your local pharmacy for proper disposal.
Affected products
| Product | Health risk |
|---|---|
| Festal Plus tablets | Labelled (in Korean) to contain ursodiol |
Issue
Health Canada is warning consumers not to use unauthorized Festal Plus tablets seized from GD Health Town, in Coquitlam (329 North Road #215), B.C., because the product is labelled to contain a prescription drug (ursodiol) and may pose serious health risks.
The unauthorized product is promoted for use in adults and children eight years of age and older who have gastrointestinal problems to help digest food; however, ursodiol is a prescription drug in Canada and is only authorized for the treatment of certain liver diseases.
Selling unauthorized health products in Canada is illegal. Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, efficacy and quality and may pose a range of serious health risks. For example, they could contain high-risk ingredients, such as prescription drugs, additives or contaminants that may or may not be listed on the label. These ingredients could interact with other medications and foods. In addition, these products may not actually contain the active ingredients that consumers would expect them to contain to help maintain and improve their health. The product is also not labelled in both English and French as required in Canada.
Prescription drugs should only be used under the advice and supervision of a health care professional because they are used to treat specific conditions and may cause serious side effects. Prescription drugs can only be legally sold to consumers in Canada with a prescription.
What you should do
- Do not use this product. Consult a health care professional if you have used this product and have health concerns.
- Return the product to your local pharmacy for proper disposal.
- Buy your prescription drugs from licensed pharmacies.
- Buy only authorized health products. Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related side effects or complaints to Health Canada.
Additional information
Background
Ursodiol, also known as ursodeoxycholic acid, is a prescription drug used for the management of cholestatic liver diseases (diseases that involve blocked or reduced bile flow from the liver). Serious side effects of taking ursodeoxycholic acid include allergic reactions, chest pain and difficulty breathing, stomach ache, nausea, diarrhea or constipation, swelling of the extremities (e.g., hands and feet), high blood pressure, fatigue, dizziness, headache, itchiness, fever and jaundice. Some patients have experienced additional symptoms such as vomiting and pain in the abdominal area caused by blockages in the gastrointestinal tract, which requires medical intervention. Blood tests are needed to monitor for the risk of liver toxicity from taking this drug. Ursodeoxycholic acid should not be used by people who have an allergy to ursodiol, have a blockage of bile flow due to liver or other disease, or by people who are pregnant, planning to become pregnant, or breastfeeding.
Details
Media and public enquiries
Media Enquiries:
Health Canada
613-957-2983
media@hc-sc.gc.ca
Public Enquiries:
613-957-2991
1-866-225-0709
info@hc-sc.gc.ca
Get notified
Receive notifications for new and updated recalls and alerts by category.