This page has been archived on the Web
Information identified as archived is provided for reference, research or recordkeeping purposes. It is not subject to the Government of Canada Web Standards and has not been altered or updated since it was archived. Please contact us to request a format other than those available.
Unauthorized sexual enhancement products seized at four Toronto retail locations may pose serious health risks
- Starting date:
- November 1, 2017
- Posting date:
- November 1, 2017
- Type of communication:
- Advisory
- Subcategory:
- Natural health products
- Source of recall:
- Health Canada
- Identification number:
- RA-64178
- Issue
- What you should do
- Who is affected
- Background
- Media enquiries
- Public enquiries
- What Health Canada is doing
- Images
Update – November 1, 2017
This advisory has been updated to reflect additional products seized from these retailers that have been tested and found to contain dangerous ingredients.
Update – October 6, 2017
This advisory has been updated to reflect additional products seized from these retailers that have been tested and found to contain dangerous ingredients.
Update – August 17, 2017
This advisory has been updated to reflect additional unauthorized products seized from these retailers.
Original Advisory – August 4, 2017
Issue
Health Canada is advising Canadians that multiple unauthorized sexual enhancement products seized from four Toronto retail locations may pose serious risks to health.
What you should do
- Stop using these products. Consult with your health care professional if you have used any of these products and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada’s Drug Product Database and Licensed Natural Health Product Database.
- Report adverse events to health products to Health Canada by calling toll-free at 1-866-234-2345, or by reporting online, by mail or by fax.
- Report complaints about health products to Health Canada by calling toll-free at 1-800-267-9675, or complete an online complaint form.
Who is affected
Consumers who have bought or used any of these products.
Affected products
The following unauthorized products were seized as they are labelled or tested and found to contain the ingredients listed below:
| Retailer | Products | Ingredient(s) |
|---|---|---|
|
The Men's Room 465 Church St. Toronto, ON |
Passion Classic | Yohimbe |
|
Hasty Market 557 Church St, Toronto, ON |
Hard Rock 3800 | Yohimbe bark extract |
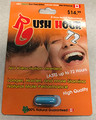
| Rush Hour 72 | Tested and found to contain sildenafil, tadalafil, desmethyl carbodenafil, and dithiodesmethyl carbodenafil (added October 6, 2017) | |
| Ginseng Red 2000 | Tested and found to contain sildenafil (added October 6, 2017) | |
| Rhino 7 Platinum 5000 | Tested and found to contain sildenafil and yohimbine (added October 6, 2017) | |
| FX12000 | Tested and found to contain sildenafil and tadalafil (added October 6, 2017) | |
|
Yeno Trading 169 The West Mall, Etobicoke, ON |
Hard Rock 3800 | Yohimbe bark extract |
| Premium Pro Power 3500 | Yohimbe bark extract | |
| Premium X Pulse 2000 | Yohimbe | |
| Dragon 69 20000 | Tested and found to contain sildenafil and tadalafil (added November 1, 2017) | |
| Krazzy Rhino Platinum 25000 | Tested and found to contain sildenafil and tadalafil (added November 1, 2017) | |
| Rhino 69 Platinum 20000 | Tested and found to contain sildenafil and tadalafil (added November 1, 2017) | |
| Rhino Fruit 15K Chewing Gum | Tested and found to contain tadalafil (added November 1, 2017) | |
| Rhino Gold 25000 | Tested and found to contain sildenafil and tadalafil (added November 1, 2017) | |
| Rhino Mint 15K Chewing Gum | Tested and found to contain tadalafil (added November 1, 2017) | |
| Triple PowerZen Platinum 7000mg | Tested and found to contain sildenafil and tadalafil (added November 1, 2017) |
The following unauthorized products were also seized by Health Canada. The Department previously seized unauthorized products with similar packaging from other retailers because they were tested and found to contain a prescription drug:
| Retailer | Products | Details |
|---|---|---|
|
Out On The Street 551 Church St. Toronto ON |
Blue Diamond | Previously tested product with similar packaging was found to contain sildenafil |
| ResErection! | Previously tested product with similar packaging was found to contain sildenafil and tadalafil | |
| Rhino | Previously tested product with similar packaging was found to contain aminotadalfil (added August 17, 2017) | |
|
The Men's Room 465 Church St. Toronto, ON |
ResErection! | Previously tested product with similar packaging was found to contain sildenafil and tadalafil |
| Rhino | Previously tested product with similar packaging was found to contain aminotadalfil (added August 17, 2017) |
Background
Sildenafil and tadalafil are prescription drug ingredients. Products containing these ingredients should be used only under the supervision of a health care practitioner. Sildenafil and tadalafil should not be used by individuals taking any kind of nitrate drug (such as nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other side effects include headache, facial flushing, indigestion, dizziness, abnormal vision and hearing loss.
Aminotadalafil is an unauthorized substance similar to tadalafil and may pose similar health risks.
Desmethyl carbodenafil and dithiodesmethyl carbodenafil are unauthorized substances similar to sildenafil and may pose similar health risks.
Yohimbine is a prescription drug and should be used only under the supervision of a health care professional. Yohimbine is derived from yohimbe, a bark extract. The use of yohimbine or yohimbe may result in serious adverse reactions, particularly in people with high blood pressure, or heart, kidney or liver disease. Side effects include increased blood pressure and heart rate, anxiety, dizziness, tremors, headache, nausea and sleep disorders. It should not be used by children, or pregnant or nursing women.
Media enquiries
Health Canada
(613) 957-2983
Public enquiries
(613) 957-2991
1-866 225-0709
What Health Canada is doing
Health Canada seized the unauthorized products from the retail locations. Should additional retailers or distributors be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Images
Select thumbnail to enlarge - opens in a new window