Importation of Italian-authorized Cisatracurio Hikma 2 mg/mL Ampoules due to the Current Shortage of Canadian-authorized Cisatracurium Omega
Summary
See Key Messages below
Affected products
Product Brand Name |
Dosage Form, Strength, Packaging Format, and Route of Administration |
Country of Authorization and Identifying Code |
Manufacturer |
Importer in Canada |
|---|---|---|---|---|
Cisatracurio Hikma 2 mg/mL |
Solution for injection/infusion, 2 mg/mL (20 mg/10 mL), 10 mL single-use clear glass ampoule with one point cut marked with a white dot, Intravenous use only |
Italy,AIC 044195093 |
Hikma Farmacêutica (Portugal), S.A. |
Hikma Canada Limited |
Issue
Given the medical necessity of Cisatracurium Omega (Cisatracurium Besylate Injection 2 mg/mL) and to maintain continuity of supply in Canada, Health Canada has authorized the exceptional, temporary importation and sale of Italian-authorized Cisatracurio Hikma 2 mg/mL with Italian-only labels by Hikma Canada Limited to mitigate the shortage.
Audience
Healthcare professionals including pharmacists, anesthesiologists, critical care physicians, emergency physicians and those involved with administering anesthesia or intubating patients.
Key messages
- Due to a shortage of Cisatracurium Omega (Cisatracurium Besylate Injection, 2 mg/mL) in Canada and given the medical necessity of this non-depolarizing neuromuscular blocking (NMB) agent, Health Canada has permitted the exceptional, temporary importation and sale of Italian-authorized Cisatracurio Hikma 2 mg/mL with Italian-only labels.
- Italian-authorized Cisatracurio Hikma 2 mg/mL is packaged in 10 mL single-use clear glass ampoules, and therefore DOES NOT have a ferrule (metal seal on vial) or cap to display the distinctive text: “WARNING: PARALYZING AGENT” or “PARALYZING AGENT” (see Appendix 1).
- Healthcare professionals are advised to:
- Be aware of important differences between the Italian-authorized Cisatracurio Hikma 2 mg/mL and the Canadian-authorized Cisatracurium Omega products (see the Information for healthcare professionals section).
- Be aware of the potential risk of errors resulting from inadvertent selection and administration of NMBs, resulting in serious harm to patients.
- Refer to the Canadian Product Monograph for the Cisatracurium Omega Single Dose (Cisatracurium Besylate Injection, 2 mg/mL) by Omega Laboratories Limited, available in English and French on Health Canada’s Drug Product Database for information on proper use.
- Be aware that the Italian-authorized Cisatracurio Hikma 2 mg/mL and the Canadian-authorized Cisatracurium Omega products should NOT be diluted in Lactated Ringer's Injection due to chemical instability.
- Proper selection of intended product must be confirmed to avoid confusion with other injectable medications available in ampoules.
Background
Cisatracurium Omega (Cisatracurium Besylate Injection, 2mg/mL) is a non-depolarizing NMB agent with an intermediate onset and duration of action indicated as an adjunct to general anesthesia, to facilitate non-emergency endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Due to a shortage of Cisatracurium Omega (Cisatracurium Besylate Injection, 2mg/mL) in Canada, Health Canada has authorized the exceptional, temporary importation and sale of Italian-authorized Cisatracurio Hikma 2 mg/mL in order to maintain continuity of supply.
Information for healthcare professionals
This drug should be administered by appropriately trained healthcare professionals, familiar with its actions, characteristics, and hazards.
In Canada, NMBs are commonly supplied in vials with distinctive text on the ferrule (metal seal on vial) and cap: “WARNING: PARALYZING AGENT” or “PARALYZING AGENT”. Canadian healthcare professionals who administer NMBs are accustomed to this labelling and packaging practice, which has been adopted by the industry as a strategy to readily identify NMBs so that they are not confused with other products.
Health Canada is aware of domestic and international reports of NMB mix-ups causing serious harm including death; some of these errors are related to changes in the labelling and packaging of these products.
Healthcare professionals should be aware that there are important differences between the Italian-authorized Cisatracurio Hikma 2 mg/mL and the Canadian-authorized Cisatracurium Omega products (see Table 1).
Table 1: Differences between Italian-authorized Cisatracurio Hikma 2 mg/mL and Canadian-authorized Cisatracurium Omega Single Dose and Cisatracurium Omega Multi-Dose
|
|
Drug product for importation |
Canadian drug product |
|
|
Product Brand Name, Dosage Form and Strength |
Cisatracurio Hikma 2 mg/mL
Solution for injection/infusion
2 mg/mL of Cisatracurium (as Cisatracurium Besylate) |
Cisatracurium Omega Single Dose
Solution for injection/infusion
2 mg/mL of Cisatracurium (as Cisatracurium Besylate)* |
Cisatracurium Omega Multi-Dose
Solution for injection/infusion
2 mg/mL of Cisatracurium (as Cisatracurium Besylate) |
|
Identifying Code
|
AIC 044195093 |
DIN 02408805 |
DIN 02408813 |
|
Packaging
|
Ampoule, with one point cut marked with a white dot |
Vial |
Vial |
|
Volume
|
10 mL |
5 mL |
10 mL |
|
Contains warning: “WARNING: PARALYZING AGENT” or “PARALYZING AGENT”
|
No Warning |
Contains Warning |
Contains Warning |
|
Contains Ferrule (metal seal on vial)
|
No |
Yes |
Yes |
|
Preservative |
Preservative-free |
Preservative-free |
benzyl alcohol 0.9% |
*This product is currently not marketed and is listed as “Dormant” on Health Canada’s Drug Product Database.
Proper selection of intended products must be confirmed to avoid confusion with other injectable medications available in ampoules.
Healthcare professionals are advised that:
- The Italian-authorized product can be used in the same manner as the Canadian-authorized Cisatracurium Omega Single Dose (Cisatracurium Besylate Injection, 2 mg/mL). Refer to the Canadian Product Monograph for the Cisatracurium Omega Single Dose (Cisatracurium Besylate Injection, 2 mg/mL) by Omega Laboratories Limited, available in English and French on Health Canada’s Drug Product Database for information on proper use.
- Hikma’s English and French translation of the Summary of Product Characteristics for Cisatracurio Hikma 2 mg/mL is available at [insert online hyperlink to Hikma’s certified English translation of the Summary of Product Characteristics for the Italian-authorized product] for reference.
- Caution should be used in breaking the glass ampoule and in aspirating the solution as per established institutional standard of care.
- The Italian-authorized Cisatracurio Hikma 2 mg/mL and the Canadian-authorized Cisatracurium Omega products should NOT be diluted in Lactated Ringer's Injection due to chemical instability.
- Additional measures to highlight that the Italian-authorized product is a NMB, such as auxiliary labels, should be considered by hospitals.
Action taken by Health Canada
To help mitigate the shortage of Cisatracurium Omega (Cisatracurium Besylate Injection, 2mg/mL) in Canada, Health Canada has permitted the exceptional, temporary importation and sale of Italian-authorized Cisatracurio Hikma 2 mg/mL by Hikma Canada Limited and has added this product to the List of Drugs for Exceptional Importation and Sale.
Health Canada has worked with Hikma Canada Limited to prepare this alert for Cisatracurium Injection. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication update will be further distributed through the MedEffect™ e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving Cisatracurio Hikma 2 mg/mL should be reported to Hikma Canada Limited or Health Canada.
Hikma Canada Limited
5995 Avebury Road, Suite 804
Mississauga, Ontario, L5R 3P9
Telephone: 1-800-656-0793
Fax: 416-485-8352
To correct your mailing address or fax number, contact Hikma Canada Limited.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Mike Armstrong
Canada Country Director
Hikma Canada Limited
Appendix 1:
A. Photos of Cisatracurio Hikma 2 mg/mL with Italian-only labelling
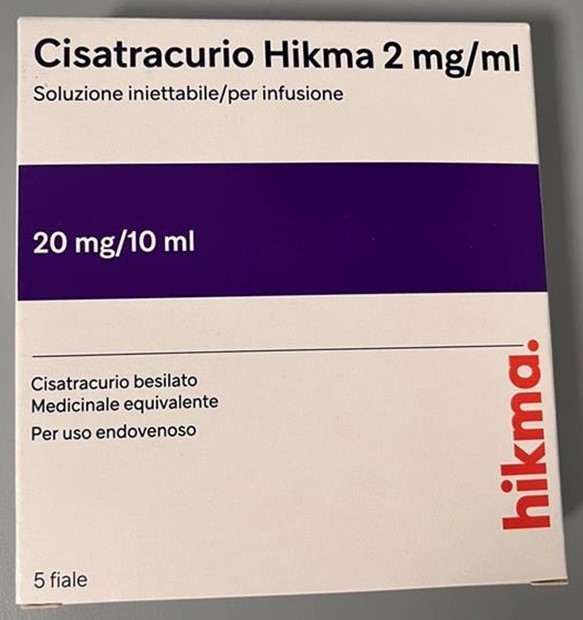

B. Cisatracurio Hikma 2 mg/mL ampoule label

C. Cisatracurio Hikma 2 mg/mL ampoule label translated in English:

D. Cisatracurio Hikma 2 mg/mL carton label:
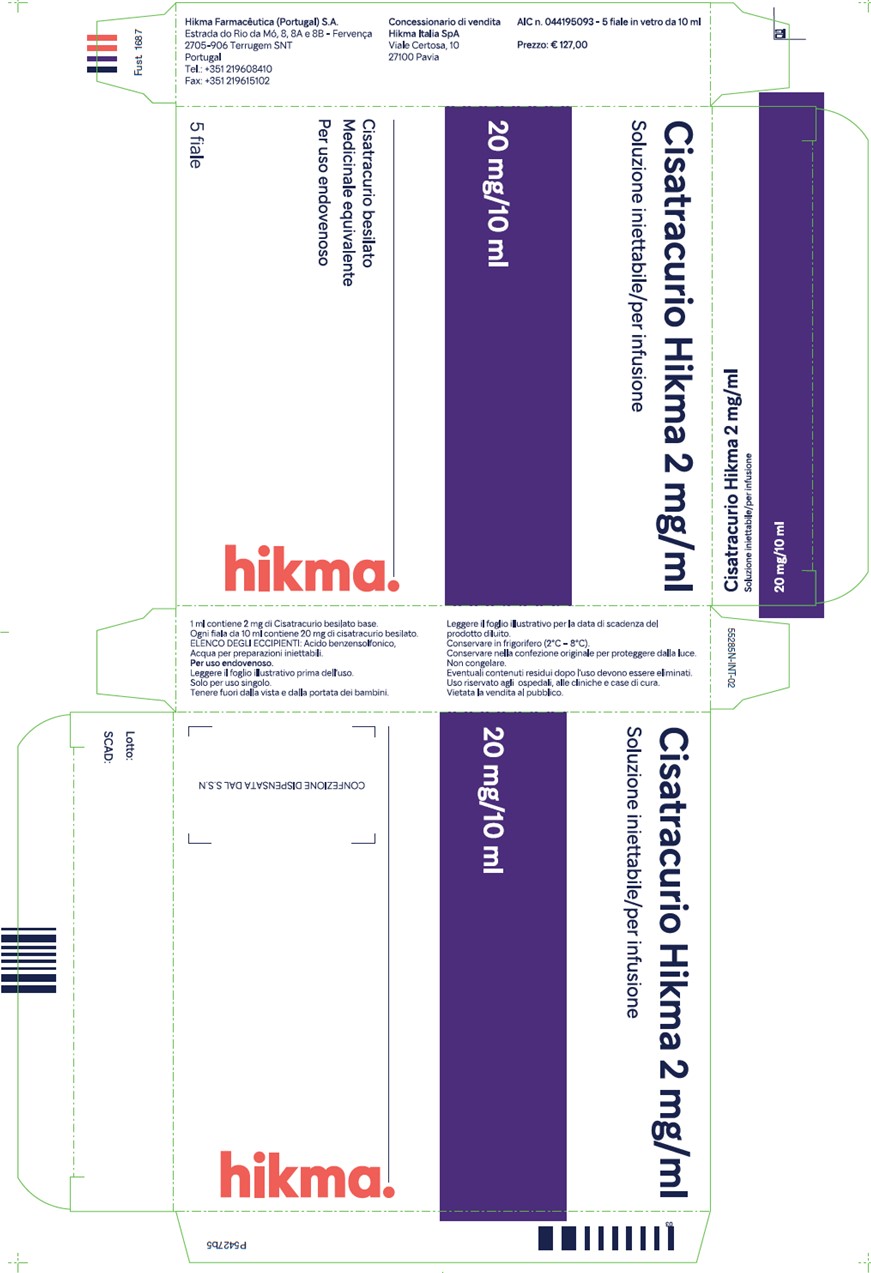
E. Cisatracurio Hikma 2 mg/mL carton label translated in English:
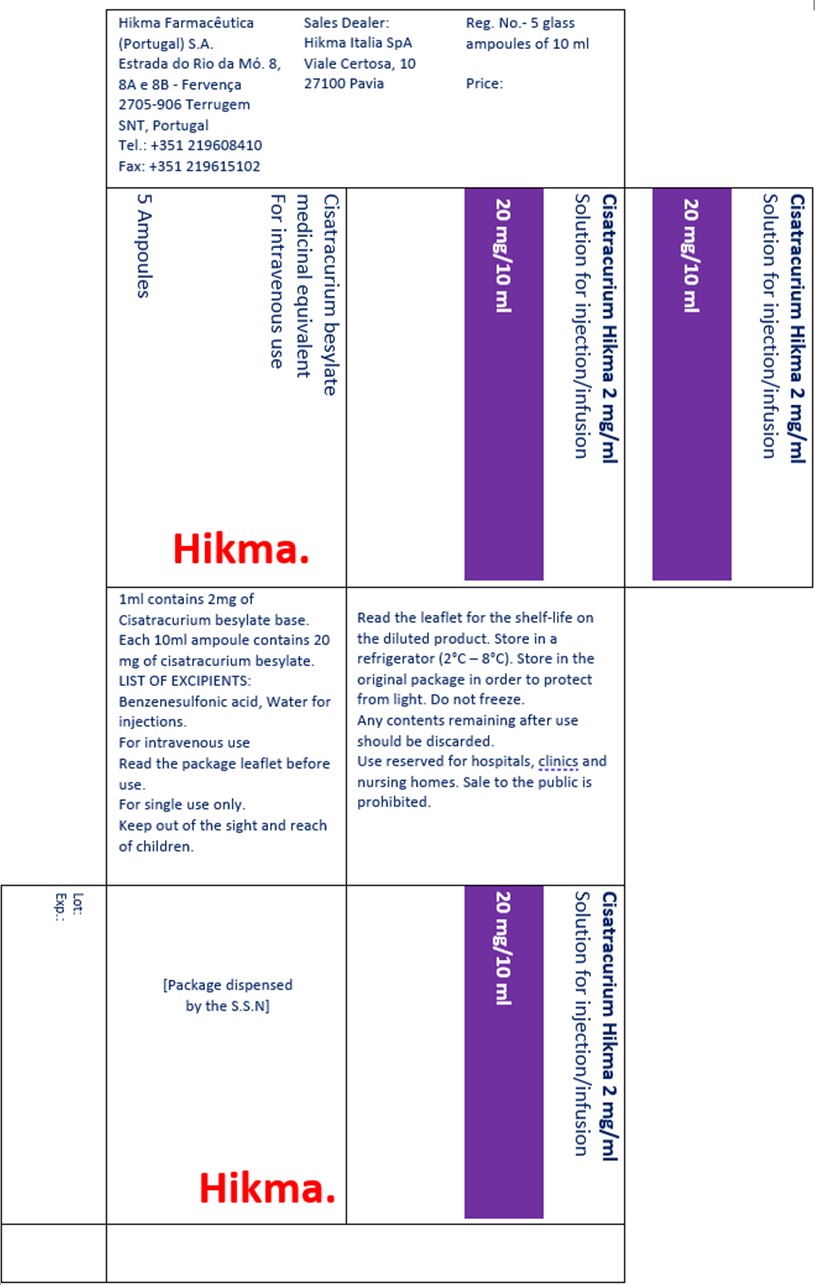
Additional information
Details
Get notified
Receive notifications for new and updated recalls and alerts by category.