Importation of Portuguese-authorized Lentocilin S 1200 1 200 000 IU / 4 mL, due to the current shortage of Canadian-authorized Bicillin L-A 1 200 000 IU / 2 mL
Summary
See Key Messages below
Affected products
| Brand Name | Dosage form and route of administration | Country of authorization and identifying code | Manufactured By | Importer in Canada |
|---|---|---|---|---|
| Lentocilin S 1200 1 200 000 IU / 4 mL | Powder and solvent for suspension for injection. Intramuscular | Portugal 9908301 | Laboratórios Atral, S.A. | Septa Pharmaceuticals Inc. |
Issue
Given the medical necessity of Bicillin L-A 1 200 000 IU / 2 mL and the need to maintain continuity of supply in Canada, Health Canada has authorized the exceptional, temporary importation and sale of Portuguese-authorized Lentocilin S 1200; 1 200 000 IU / 4 mL, manufactured by Laboratórios Atral, S.A. with English-only labels, by Septa Pharmaceuticals Inc., to mitigate the shortage.
Lentocilin S has the same active ingredient, strength (1 200 000 IU), and route of administration (intramuscular) as Bicillin L-A. However, there are differences in the formulation, dosage form, reconstitution requirement, and presentation between Lentocilin S and Bicillin L-A. Healthcare professionals are advised to enhance the safety monitoring of the imported drug, including monitoring for potential adverse effects.
Audience
Healthcare professionals including physicians, nurses, hospital pharmacists, public health and sexual health clinics, group purchasing organizations, and wholesalers.
Key messages
- Due to a critical shortage of the antibiotic Bicillin L-A 1 200 000 IU / 2 mL in Canada, and given the medical necessity of this drug, Health Canada has permitted the exceptional, temporary importation and sale of Portuguese-authorized Lentocilin S 1200; 1 200 000 IU / 4 mL with English-only labels.
- Lentocilin S has the same active ingredient, strength (1 200 000 IU) and route of administration (intramuscular) as Bicillin L-A. However, Lentocilin S has a different formulation, dosage form, reconstitution requirement, and presentation than Bicillin L-A (see Appendix 1).
- Healthcare professionals are advised to:
- Be aware that the active ingredient (benzathine benzylpenicillin) in Lentocilin S is identical to the active ingredient (penicillin G benzathine) in Bicillin L-A. The products use different nomenclature on their labels.
- Be aware that Lentocilin S is supplied as a powder in a vial, to be reconstituted with the co-packaged diluent containing lidocaine hydrochloride 1.5% (final volume 4 mL), whereas Bicillin L-A is supplied as a suspension in a ready-to-use pre-filled syringe and does not contain lidocaine (final volume 2 mL) (see the Information for healthcare professionals section).
- Be aware that Lentocilin S contains soy phospholipids and may cause hypersensitivity reactions (urticaria, anaphylactic shock) in patients with a history of allergy to soybeans.
- Refer to the indications and the Serious Warnings and Precautions Box, including information related to severe cutaneous adverse reactions (SCAR) associated with penicillin G benzathine, in the Canadian Product Monograph for Bicillin L-A from Pfizer Canada ULC, available in English and French on Health Canada’s Drug Product Database.
- Refer to the Lentocilin S Summary of Product Characteristics (SmPC) in English and French for product specific information on dosing, preparation and administration, warnings and precautions, and storage and handling.
Background
Bicillin L-A (intramuscular penicillin G benzathine) is indicated in the treatment of infections due to penicillin-G-sensitive microorganisms that are susceptible to the low and very prolonged serum levels common to this particular dosage form. Therapy should be guided by bacteriological studies (including sensitivity tests) and by clinical response.
Currently, there is a critical shortage of Canadian-authorized Bicillin L-A 1 200 000 IU / 2 mL in Canada. To help mitigate the shortage, Health Canada has permitted the exceptional, temporary importation and sale of Portuguese-authorized Lentocilin S 1200; 1 200 000 IU / 4 mL, with English-only labels.
Information for healthcare professionals
Lentocilin S has the same active ingredient, strength (1 200 000 IU), and route of administration (intramuscular) as Bicillin L-A. However, there are differences in the formulation, dosage form, reconstitution requirement, and presentation among other characteristics, which are important to note (see Table 1).
|
|
Portuguese drug product for importation |
Canadian drug product |
|---|---|---|
|
Product Brand Name, Dosage Form and Strength |
Lentocilin S 1200 (Laboratórios Atral, S.A., Portugal)
Each vial contains 1 200 000 IU of benzathine benzylpenicillin 1 200 000 IU / 4 mL, Powder and solvent for suspension. |
Bicillin L-A (Pfizer Canada ULC)
penicillin G benzathine 1 200 000 IU per 2 mL suspension for injection. |
|
Identifying Code
|
9908301 |
DIN 02291924 |
|
Product Presentation |
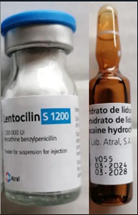
Each unit consists of a clear glass vial for injection (powder) and an amber glass ampoule with diluent. |
2 mL ready-to-use pre-filled syringe. |
|
Reconstitution |
Reconstitute immediately prior to use.
Refer to Lentocilin S SmPC for instructions. |
None |
|
Administration |
In infants younger than 2 years, and if considered necessary, the dosage may be divided and administered in two separate sites.
A needle with a minimum 18 gauge is required to administer Lentocilin S. |
No reference to divided dosage in infants. |
|
Additional Medicinal and Non-Medicinal Ingredients requiring caution |
Lidocaine hydrochloride (present in the ampoule containing the diluent) is identified as a non-medicinal ingredient in the Lentocilin S SmPC - it should be used with caution in specific populations such as the presence of cardiovascular, hepatic or renal dysfunction, inflammation and/or infection at the injection site or sensitivity to local anesthetics of the amide type; children, the elderly and patients with acute illnesses or debilitated; and patients on concomitant central nervous system (CNS) depressant drugs.
Soy phospholipids - may cause hypersensitivity reactions (urticaria, anaphylactic shock) in patients with a history of allergy to soybeans. |
None |
|
Storage |
Store below 25ºC. Store in the original package in order to protect from light and moisture. |
Store under refrigeration (2o-8oC). May be removed from refrigerator and stored for 7 days at a temperature not exceeding 30oC. Protect from freezing. |
Healthcare professionals are advised that:
- Due to the different product formulation, enhanced monitoring of the safety of the imported drug for the intended disease condition is recommended, including monitoring for potential adverse effects.
- Lentocilin S contains soy phospholipids and may cause hypersensitivity reactions (urticaria, anaphylactic shock) in patients with a history of allergy to soybeans. The co-packaged diluent contains lidocaine hydrochloride and should be used with caution in specific populations, including those with sensitivity to local anesthetics of the amide type.
- The reconstituted Lentocilin S product should be administered immediately after reconstitution. Note that there is a difference in the volume for injection of the reconstituted Lentocilin S (4 mL) compared to Bicillin L-A (2 mL).
- A needle with a minimum 18 gauge is required to administer Lentocilin S.
- Refer to the indications and the Serious Warnings and Precautions Box, including information related to severe cutaneous adverse reactions (SCAR), in the Canadian Product Monograph for Bicillin L-A from Pfizer Canada ULC, available in English and French on Health Canada’s Drug Product Database.
- Refer to the Lentocilin S Summary of Product Characteristics (SmPC) in English and French for product specific information on dosing, preparation and administration, warnings and precautions, and storage and handling.
- Aspects of the labels of Lentocilin S may differ from Bicillin L-A (see images of Lentocilin S in Appendix 1). Proper selection of the intended product must be verified to avoid confusion with other products and to prevent medication errors.
- Lentocilin S does not have a Drug Identification Number (DIN) or a barcode that scans in medication management systems in Canada. A facility-generated sticker may be required to enable barcode scanning and allow the product being dispensed and administered to be properly identified.
Action taken by Health Canada
Health Canada has worked with Septa Pharmaceuticals Inc. to prepare this alert for Portuguese-authorized Lentocilin S 1200; 1 200 000 IU / 4 mL. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication will be further distributed through the MedEffect™ e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related side effects depends on health care professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving Lentocilin S should be reported to Septa Pharmaceuticals Inc. or Health Canada.
Septa Pharmaceuticals Inc.
7035 Maxwell Road, Unit 2
Mississauga, ON
L5S 1R5
Canada
Telephone: +1 905-564-5665
Fax: +1 905-564-1291
To correct your mailing address or fax number, contact Septa Pharmaceuticals Inc.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Devinder Kumar
President & CEO
Septa Pharmaceuticals Inc.
Appendix 1
Portuguese-authorized Lentocilin S 1200; 1 200 000 IU / 4 mL, carton, vial, and ampoule labels:
Lentocilin S 1200; 1 200 000 IU / 4 mL, Carton Label:
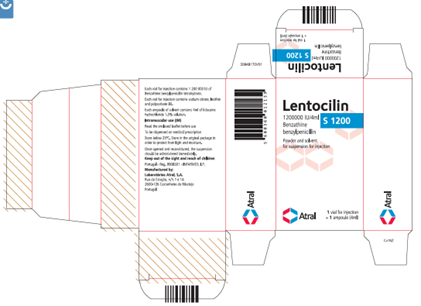
Each vial for injection contains 1 200 000 IU of
Benzathine benzylpenicillin tetrahydrate.
Each vial for injection contains sodium citrate, lecithin
and polysorbate 80.
Each ampoule of solvent contains 4ml of lidocaine
hydrochloride 1.5% solution.
Intramuscular use (IM)
Read the enclosed leaflet before use
To be dispensed on medical prescription
Store below 25°C. Store in the original package in
order to protect from light and moisture.
Once opened and reconstituted, the suspension
should be administered immediately.
Keep out of the sight and reach of children
Portugal – Reg. 9908301 – INFARMED, I.P.
Manufactured by:
Laboratórios Astral, S.A.
Rua da Estação, n.°s 1 e 1A
2600-726 Castanheira do Ribatejo
Portugal
30848170V01
Atral
Lentocilin 
1 200 000 IU/4ml
Benzathine benzylpenicillin
1 vial for injection
+ 1 ampoule (4ml)
Lentocilin 
1 200 000 IU/4ml
Benzathine benzylpenicillin
Powder and solvent for suspension for injection
Atral
1 vial for injection
+ 1 ampoule (4ml)
Atral
Lentocilin S 1200; 1 200 000 IU / 4 mL, Vial and Ampoule Image:
Lentocilin S 1200; 1 200 000 IU / 4 mL, Vial Label:
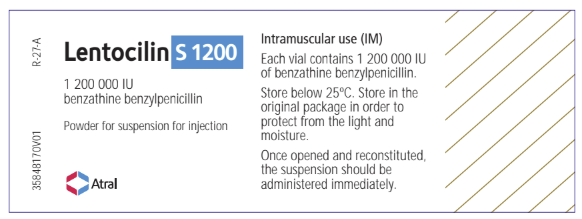
Lentocilin 
1 200 000 IU
benzathine benzylpenicillin
Powder for suspension for injection
Atral
Intramuscular use (IM)
Each vial contains 1 200 000 IU
of benzathine benzylpenicillin.
Store below 25°C. Store in the
original package in order to protect from the light
and moisture.
Once opened and reconstituted,
the suspension should be
administered immediately.
Lentocilin S 1200; 1 200 000 IU / 4 mL, Ampoule Label:

Cloridrato de lidocaina1,5%
Clorhidrato de lidocaine 1,5%
Lidocaine hydrochloride 1.5%
IM
Lab. Atral, S.A. PORTUGAL 4ml
Lote:
Prep.:
EXP: 220 . 311185.V01
What you should do
See Key Messages below
Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.