Importation of UK-authorized Hypurin Porcine Neutral Insulin Injection
Summary
See Key Messages below
Affected products
Hypurin Porcine Neutral Insulin Injection, 100 IU / mL, (L) PY40093, Exp 31 May 2026.
Issue
The last of the Canadian-labelled supply of Hypurin Regular Insulin, Pork has a December 2025 expiration date. Health Canada has authorized the exceptional, temporary importation and sale of UK-authorized Hypurin Porcine Neutral to extend the availability of pork insulin in Canada to May 2026. The UK-authorized product may be used as a substitute for the Canadian-authorized Hypurin Regular Insulin, Pork.
Audience
Healthcare professionals including pharmacists, nurse practitioners, family physicians, and endocrinologists / diabetologists.
Key messages
- Due to the discontinuation of Hypurin Regular Insulin, Pork in Canada1, and to extend the availability of pork insulin to May 2026, Health Canada has authorized the exceptional, temporary importation and sale of UK-authorized Hypurin Porcine Neutral with English-only labels by Wockhardt UK Ltd.
- The UK-authorized product, Hypurin Porcine Neutral, and the Canadian-authorized product, Hypurin Regular Insulin, Pork are:
- short acting insulins;
- the same product formulation; and
- produced using the same manufacturing processes at the same facility, and packaged using the same primary packaging components.
- Hypurin Porcine Neutral is interchangeable with Hypurin Regular Insulin, Pork and can be used in the same manner. The products are identical, with the exception of important differences between the UK and Canadian labelling.
- Healthcare professionals are advised to:
- Be aware that there are important differences in product labelling between the UK-authorized product and the Canadian-authorized product, including product naming and differences in expression of strength. In addition, the UK labels are in English only and lack the word PORK to alert users that this is an animal sourced insulin (see Information for healthcare professionals section).
- Refer to the Canadian Product Monograph for Hypurin Regular Insulin, Pork by Wockhardt UK Ltd (DIN 02275872), available in English and French on Health Canada’s Drug Product Database, for information on product use.
Background
There are two types of insulin used to manage diabetes:
- Animal-sourced insulin (porcine)
- Human insulin and insulin analogues
In Canada, Hypurin is indicated for:
- Treatment of patients with insulin-dependent diabetes mellitus who are already using an animal derived insulin.
- Treatment of new patients with insulin-dependent diabetes mellitus who are unable to tolerate recombinant human insulin or whose diabetes is inadequately controlled by recombinant human insulin.
Wockhardt UK, Ltd, the sole supplier of porcine insulin, is no longer able to supply porcine insulin vials for the Canadian market, as the vial production line used for filling Porcine Insulin Injection has been decommissioned.
With the discontinuation of animal-sourced insulin in Canada, patients will need to speak with their healthcare professional if they have questions or concerns about this discontinuation, and to discuss alternative treatments. Current inventory of Canadian-authorized Hypurin Regular Insulin, Pork (short acting) expires in December 2025. The exceptional importation of Hypurin Porcine Neutral extends the availability of the product in Canada for the short-term. The UK-authorized supply expires in May 2026.
There is inventory of Canadian-authorized Hypurin NPH (intermediate acting) that expires in April 2026, therefore, Wockhardt UK, Ltd will not be importing UK-authorized inventory of the intermediate acting insulin at this time.
No additional inventory of Hypurin vials (short acting or intermediate acting) will be available after the existing inventory has been depleted or has expired.
Information for consumers
Hypurin Porcine Neutral (UK product) is interchangeable with and equivalent to Hypurin Regular Insulin, Pork (Canadian product). In addition to the product names being different, patients should be aware that there are important differences in product labelling between the UK product and the Canadian product, including:
- The UK labels are in English only.
- The units used to represent medicinal ingredient strength appear as “IU / ml” on the UK labels, but this is considered to be the same as the “units per ml” that is displayed on the Canadian labels.
- The UK labels are missing the word PORK, although the product name Hypurin Porcine Neutral includes the word Porcine to let users know that this insulin is from an animal source.
Patients should contact their healthcare professional for information about labelling differences between the two products.
For patients who use Canadian-authorized Hypurin NPH Insulin Isophane Pork (intermediate acting) along with Canadian-authorized Hypurin Regular Insulin, Pork (short acting) to manage their diabetes, the Canadian-authorized Hypurin NPH Insulin Isophane Pork (intermediate acting) and the UK-authorized Hypurin Porcine Neutral (short acting) may be used together if prescribed in that manner. Patients should contact their healthcare professional for more information on using insulin products together.
Information for healthcare professionals
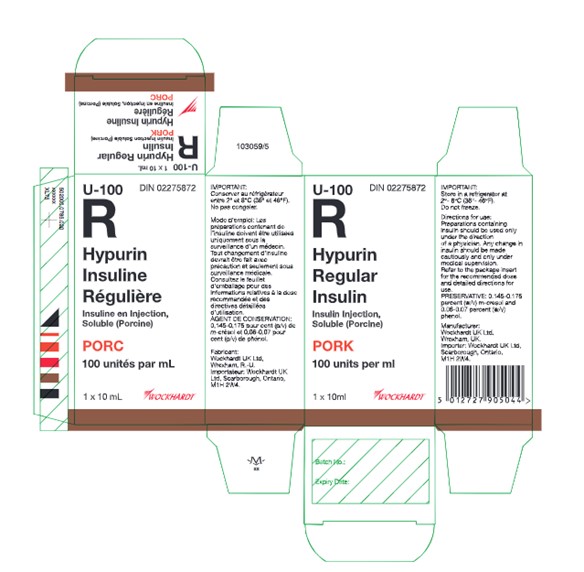
The UK-authorized product has the same active ingredient, strength and dosage form as the Canadian-authorized product. Both the UK-authorized product and the Canadian-authorized product are packaged in the same 10 mL vials (see Appendix 1). However, there are important differences in labelling between the UK-authorized product and Canadian-authorized product, including product naming and differences in expression of strength.
Healthcare professionals are advised of the following key differences on the UK carton, and vial label (see Appendix 1):
- The UK product name, Hypurin Porcine Neutral, is not consistent with the Canadian product name, Hypurin Regular Insulin, Pork. However, these two products are interchangeable.
- The UK labels are only in English and lack French text.
- The units used to represent the medicinal ingredient strength on the UK product labels appear as “IU / ml”. This is considered the same as the “units per ml” that represents the medicinal ingredient strength on the Canadian labels.
- PORK is clearly indicated in red on the Canadian labels to alert users that this is an animal sourced insulin. This additional annotation is missing on the UK product label although the Porcine source of the insulin is included as part of the product name.
- The route of administration and the dosage form is indicated on the inner and outer labels of the UK products, whereas this is not indicated on the Canadian labels.
Additionally, the UK-authorized product does not have a Drug Identification Number (DIN) or a barcode that scans in medication management systems in Canada. A facility-generated sticker is recommended to enable barcode scanning and allow accurate identification of the product being dispensed and administered.
Action taken by Health Canada
Health Canada is working with patient and healthcare professional associations to communicate the discontinuation of animal-sourced insulin products.
Health Canada has worked with Wockhardt UK, Ltd to prepare this alert for UK-authorized Hypurin Porcine Neutral. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication update will be further distributed through the MedEffectTM e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related adverse reactions depends on healthcare professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving Hypurin Porcine Neutral should be reported to Wockhardt UK, Ltd or Health Canada.
Wockhardt UK, Ltd
2000 Ellesmere Rd, Unit 16,
Scarborough, ON
M1H 2W4
Canada
Tel: (416) 438-6727 or 1-800-539-0801
Fax: (416) 438-3179
To correct your mailing address or fax number, contact Wockhardt UK Ltd.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Lead Directorate: Regulatory Operations and Enforcement Branch
E-mail: (Generic e-mail address): hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Harry Jones
Export Officer
References
1. Notice: Animal-sourced insulin discontinuation, Health Canada, published March 18, 2025 [https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/drug-shortages/information-consumers/supply-notices/animal-sourced-insulin-discontinuation.html]
Appendix 1
Image of UK-authorized vial label of Hypurin Porcine Neutral
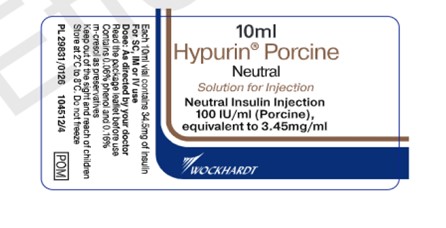
Image of UK-authorized carton label of Hypurin Porcine Neutral
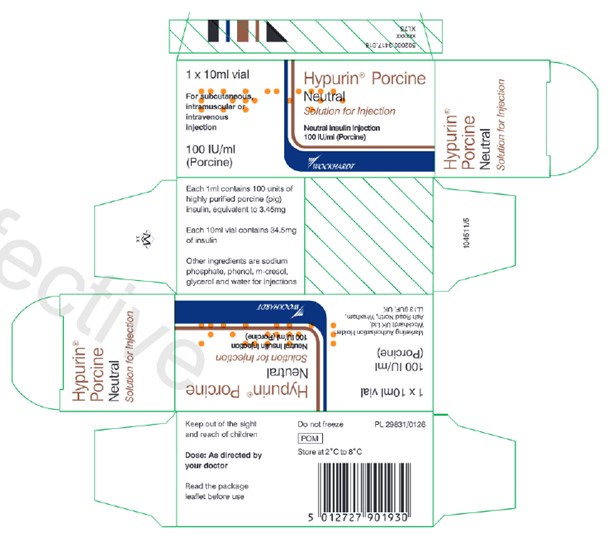
Image of Canadian-authorized vial label of Hypurin Regular Insulin Pork
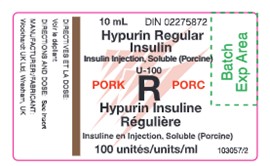
Image of Canadian-authorized carton label of Hypurin Regular Insulin Pork
Additional information
Details
Get notified
Receive emails about new and updated recall and safety alerts.