Importation of USA-authorized Carbamazepine Extended-Release Tablets, USP 200 mg and 400 mg due to the current shortage of Canadian-authorized carbamazepine
Summary
See Key Messages below
Affected products
Brand name |
Dosage form and route of |
Country of authorization and |
Manufacturer |
Importer in Canada |
|---|---|---|---|---|
Carbamazepine Extended-Release Tablets, USP 200 mg |
Tablets Oral |
USA NDC: |
Ingenus Pharmaceuticals, LLC |
Septa Pharmaceuticals Inc. |
Carbamazepine Extended-Release Tablets, USP 400 mg |
Tablets Oral |
USA NDC: |
Ingenus Pharmaceuticals, LLC |
Septa Pharmaceuticals Inc. |
Issue
Given the medical necessity of carbamazepine extended-release tablets and the need to maintain continuity of supply in Canada, Health Canada has authorized the exceptional, temporary importation and sale of USA-authorized Carbamazepine Extended-Release Tablets, USP manufactured by Ingenus Pharmaceuticals, LLC, with English-only labels, by Septa Pharmaceuticals Inc., to mitigate the shortage.
The USA-authorized product has the same active ingredient (carbamazepine), strength (200 mg and 400 mg), dosage form (extended-release tablets), and route of administration (oral) as the Canadian-authorized product. However, there are differences in formulation and physical appearance between the USA-authorized and Canadian-authorized products. Healthcare professionals are advised to enhance their monitoring of the safety and effectiveness for the imported drug.
Audience
Healthcare professionals including neurologists, psychiatrists, family physicians, hospital pharmacists and community pharmacies, group purchasing organizations, and wholesalers.
Key messages
- Due to a shortage of carbamazepine extended-release tablets in Canada and given the medical necessity of this anticonvulsant drug, Health Canada has permitted the exceptional, temporary importation and sale of USA-authorized Carbamazepine Extended-Release Tablets, USP with English-only labels.
- This USA-authorized product has the same active ingredient (carbamazepine), strength (200 mg and 400 mg), dosage form (extended-release tablets), and route of administration (oral) as the Canadian-authorized product.
- However, the USA-authorized product has a different formulation than the Canadian-authorized product, and its bioequivalence to the Canadian-authorized product has not been confirmed. Also, this product has a different physical appearance than the Canadian-authorized product (see Appendix 1).
- Healthcare professionals are advised to:
- Enhance their monitoring of the safety and effectiveness for the imported drug for the intended disease condition since bioequivalence is unconfirmed and carbamazepine is considered to have a narrow therapeutic index. Careful follow-up and potential blood test monitoring must be undertaken during the product switch.
- Be aware of the differences in physical appearance between the USA- and Canadian-authorized products (see the Information for healthcare professionals section).
Background information
Carbamazepine is an anticonvulsant drug indicated for the treatment of epilepsy, the symptomatic relief of trigeminal neuralgia, and the treatment of acute mania and prophylaxis in bipolar (manic-depressive) disorders.
Currently, there is a critical shortage of Canadian-authorized carbamazepine extended-release tablets 200 mg and 400 mg in Canada. To help mitigate the shortage, Health Canada has permitted the exceptional, temporary importation and sale of USA-authorized Carbamazepine Extended-Release Tablets, USP 200 mg and 400 mg, with English-only labels.
Information for consumers
Carbamazepine is used to treat certain types of seizures and acute mania or bipolar disorder. It is also used to relieve pain caused by trigeminal neuralgia. Consumers should contact their healthcare professional for more information concerning this communication.
Carbamazepine has a narrow therapeutic index, which means that there is only a small difference between the minimum effective dose and the minimum toxic dose.
If the level of carbamazepine in the body is too low, a person’s medical condition might not be adequately controlled. A person with a history of seizures may experience an increase in the number of seizures, or epileptic episodes. Those who are taking carbamazepine to treat pain from trigeminal neuralgia or another painful condition, may experience a decrease in their pain control. Those taking carbamazepine to treat mania or bipolar disorders may show symptoms of a manic episode. If any of these events occur, the patient should be evaluated by a physician to ensure that they are receiving the right dose of carbamazepine.
If the level of carbamazepine in the body is too high, a person may experience symptoms of carbamazepine intoxication including tremor, restlessness, drowsiness, agitation, hallucination, disorientation, reduced level of consciousness, blurred vision, slurred speech, abnormal movements, slow breathing, fast heart beats, high or low blood pressure, dizziness, nausea, vomiting, or reduced urine output. Patients experiencing such symptoms should be evaluated by a physician in a hospital setting to ensure that the level of carbamazepine is correct.
Patients should discuss any questions or concerns about this information with their healthcare professional. Patients should continue to inform their healthcare professional if they are experiencing any side effects while receiving carbamazepine.
Information for healthcare professionals
The approved USA-authorized product has the same active ingredient (carbamazepine), strength (200 mg and 400 mg), dosage form (extended-release tablets), and route of administration (oral) as the Canadian-authorized product.
The USA-authorized product, however, differs from the Canadian-authorized product in terms of the appearance as listed in the table below.
|
|
USA-authorized product |
Canadian-authorized product |
|
200 mg |
Tablets are white in color, round in shape and 10 mm in size, without score, with the imprint code I258. |
Tablets are beige-orange, oval and slightly biconvex. C/G engraved on one side and H/C engraved on the other. Fully bisected on both the sides. |
|
400 mg |
Tablets are white in color, round in shape and 12 mm in size, without score, with the imprint code I259. |
Tablet is brown-orange, oval and slightly biconvex. CG/CG engraved on one side and ENE/ENE engraved on the other. Fully bisected on both the sides. |
Healthcare professionals are advised that:
- The USA-authorized product has a different formulation than the Canadian-authorized product, and its bioequivalence to the Canadian-authorized product has not been confirmed. Carbamazepine is considered to have a narrow therapeutic index, exhibiting a narrow difference between therapeutic and toxic plasma concentrations. In general, interchanging between non-bioequivalent products that have a narrow therapeutic index may result in patients being exposed to minor variations in drug concentrations, which may affect the effectiveness or the safety of the drug.
- Enhanced monitoring of the safety and effectiveness for the imported drug for the intended disease condition is recommended. Careful follow-up and potential blood test monitoring must be undertaken during the product switch.
- The USA-authorized product can be used in the same manner as the Canadian-authorized product. Refer to the Canadian Product Monograph for Tegretol CR (carbamazepine extended-release tablets) 200 mg and 400 mg from Novartis Pharmaceuticals Canada Inc., available in English and French on Health Canada’s Drug Product Database, for information on the appropriate use of the product, including the Indications, Contraindications, Warnings and Precautions, Adverse Reactions, Dosage and Administration and Storage Conditions.
- USA-authorized Carbamazepine Extended-Release Tablets, USP must be swallowed whole and never crushed or chewed.
- Aspects of the label on the bottle of the USA-authorized product may differ from the Canadian-authorized product (see images of the USA-authorized product in Appendix 1). Proper selection of the intended product must be verified to avoid confusion with other products and to prevent medication errors.
- The USA-authorized product does not have a Drug Identification Number (DIN) or a barcode that scans in medication management systems in Canada. A facility-generated sticker may be required to enable barcode scanning and allow the product being dispensed and administered to be properly identified.
- Information about USA-authorized Carbamazepine Extended-Release Tablets, USP 200 mg and 400 mg for reference by healthcare professionals is available (in English only) at https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=de67de85-15b3-4c27-b604-d028ce3789d0.
Action taken by Health Canada
To help mitigate the shortage of carbamazepine extended-release tablets 200 mg and 400 mg in Canada, Health Canada has permitted the exceptional, temporary importation and sale of USA-authorized Carbamazepine Extended-Release Tablets, USP 200 mg and 400 mg and has added this product to the List of Drugs for Exceptional Importation and Sale.
Health Canada has worked with Septa Pharmaceuticals Inc. to prepare this alert for Carbamazepine Extended-Release Tablets, USP. Health Canada is communicating this important safety information to healthcare professionals and Canadians via the Recalls and Safety Alerts Database on the Healthy Canadians Web Site. This communication update will be further distributed through the MedEffect™ e-Notice email notification system.
Report health or safety concerns
Managing marketed health product-related side effects depends on healthcare professionals and consumers reporting them. Any case of serious or unexpected side effects in patients receiving USA-authorized Carbamazepine Extended-Release Tablets, USP should be reported to Septa Pharmaceuticals Inc. or Health Canada.
Septa Pharmaceuticals Inc.
7035 Maxwell Road, Unit 2
Mississauga, ON
L5S 1R5
Canada
Telephone: +1 905-564-5665
Fax: +1 905-564-1291
To correct your mailing address or fax number, contact Septa Pharmaceuticals Inc.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting for information on how to report online, by mail or by fax.
For other health product inquiries related to this communication, contact Health Canada at:
Regulatory Operations and Enforcement Branch
E-mail: hpce-cpsal@hc-sc.gc.ca
Telephone: 1-800-267-9675
Original signed by
Devinder Kumar
President & CEO
Septa Pharmaceuticals Inc.
Appendix 1
USA-authorized Carbamazepine Extended-Release Tablets, USP 200 mg and 400 mg Bottles:
Carbamazepine Extended-Release Tablets, USP 200mg
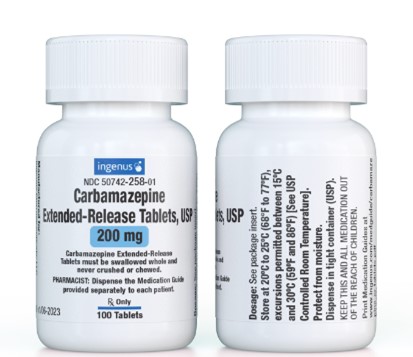

Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811
Ingenus
NDC 50742-258-01
Carbamazepine
Extended-Release Tablets, USP
200 mg
Carbamazepine Extended-Release
Tablets must be swallowed whole and
never crushed or chewed.
PHARMACIST: Dispense the Medication Guide
provided seperately to each patient.
Rx Only
100 Tablets
258-01 v1/06-2023
Dosage: See package insert.
Store at 20°C to 25°C (68°F to 77°F),
excursions permitted between 15°C
and 30°C (59°F and 86°F) [See USP
Controlled Room Temperature].
Protect from moisture.
Dispense in tight container (USP).
KEEP THIS AND ALL MEDICATION OUT
OF THE REACH OF CHILDERN.
Print Medication Guides at:
http://www.ingenus.com/medguide/carbamazepine-er-tablets.pdf
513300
350742258017
Carbamazepine Extended-Release Tablets, USP 400 mg
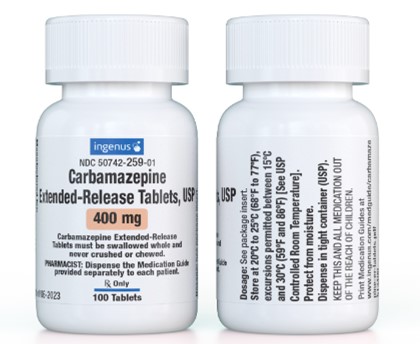

Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32811
Ingenus
NDC 50742-259-01
Carbamazepine
Extended-Release Tablets, USP
400 mg
Carbamazepine Extended-Release
Tablets must be swallowed whole and
never crushed or chewed
PHARMACIST: Dispense the Medication Guide
provided seperately to each patient.
Rx Only
100 Tablets
259-01 v1/06-2023
Dosage: See package insert.
Store at 20°C to 25°C (68°F to 77°F),
Excursions permitted between 15°C
and 30°C (59°F and 86°F) [See USP
Controlled Room Temperature].
Protect from moisture.
Dispense in tight container (USP).
KEEP THIS AND ALL MEDICATION OUT
OF THE REACH OF CHILDERN.
Print Medication Guides at:
www.ingenus.com/medguide/carbamazepine-er-tablets.pdf
513400
350742259014
USA-authorized Carbamazepine Extended-Release Tablets, USP 200 mg and 400 mg Tablets:
Carbamazepine Extended-Release Tablets, USP 200mg

Carbamazepine Extended-Release Tablets, USP 400mg

Additional information
Details
Get notified
Receive notifications for new and updated recalls and alerts by category.